O.W.F. Natan, K.P. Bouter.
Oscar Natan
Hubrecht Institute
Uppsalalaan 8
3584 CT, Utrecht
Introduction: Metformin is the first-line drug in the treatment of patients with type 2 diabetes mellitus (T2DM). Unfortunately, gastrointestinal adverse events are common, with a prevalence ranging between 0-60% and 11.4-16.1% of the patients decide to stop treatment with metformin Immediate release (IR) because of metformin intolerance (1-6). The Gastrointestinal Symptom Rating Scale (GSRS) is a verified tool to objectivate gastrointestinal complaints. (7)
Study design: An open-label, multicentre study (n=28) with T2DM patients with a GSRS-score ≥ 29. After inclusion, patients with a GSRS-score ≥ 29 were switched immediately from metformin IR to ER and after 4 weeks the GSRS-questionaire will be performed. Previous research suggests that patients with a GSRS-score ≥ 29 could benefit witch a switch. (7)
Objectives: The goal of this study is to investigate if a switch from metformin IR to metformin ER could reduce gastrointestinal complaints. The (GSRS) was used to evaluate the change in gastrointestinal complaints. Patients with T2DM, who had been using metformin for at least 4 weeks, with a GSRS-total score ≥ 29 were convert to metformin ER at a dosage similar to that of their treatment with metformin IR. Metformin ER was given once daily.
Results: From 3 participating site locations, a total of 217 patients were screened on the inclusion criteria; 28 of them scored 29 or higher (12,9%) and were suggested to change their treatment to metformin ER same dose. The mean GSRS-score at inclusion with metformin IR was 45,3 and 4 weeks after switch to metformin ER the mean GSRS-score was 25,8. A Paired Samples T Test calculated a decrease of 19,6 in GSRS-score with a significancy p < 0,01.
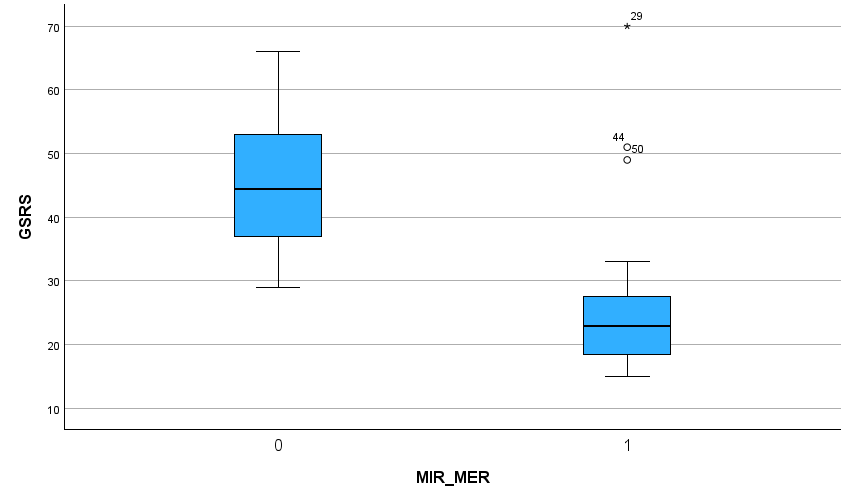
Figure 1: Boxplot – 0 (Metformin IR; during inclusion), 1 (Metformin ER; 4 weeks after switch)
Conclusion: The percentage of 12,9% seems to be in line with literature data about gastrointestinal adverse events. The T2DM guidelines in the Netherlands suggest to lower the dose of metformin (or cancel metformin treatment) and add or replace a sulfonyl derivate or if not effective, change to insulin therapy or try a DPP4-antagonist or a GLP1-receptor antagonist.
Discussion: This study shows that a switch to a metformin extended release formulation seems to decrease the gastrointestinal adverse events based on the GSRS-score in almost all patients and prevent them from stopping the metformin treatment. From the two patients, whose GSRS-score did not decreased from a switch to metformin ER, it might be because of co-medication like sulfonyl derivates. These medicines are known for gastrointestinal adverse events.
Besides the decrease of adverse events, metformin ER could increase the compliance due to a 1-daily dose instead of 2 or 3 times a day, is cheaper than alternative treatments and the dose of metformin could be increased for a more effective treatments of glucose regulation.
References:
1. Dandona P, Fonseca V, Mier A, Beckett AG (1983) Diarrhea and metformin in a diabetic clinic. Diabetes Care. 6(5): 472-474.
2. UKPDS. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 352(9131): 854-865.
3. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, et al. (2006) Glycaemic durability of rosiglitazone, metformin, or glyburide monotherapy. New England Journal of Medicine. 355(23): 2427-2443.
4. Florez H, Luo J, Castillo-Florez S, Mitsi G, Hanna J, et al. (2010) Impact of metformin-induced gastro-intestinal symptoms on quality of life and adherence in patients with type 2 diabetes. Postgraduate Medicine. 122(2): 112-120.
5. de Jong L, Härmark L, van Puijenbroek E (2016) Time course, outcome and management of adverse drug reactions associated with metformin from patient’s perspective: a prospective, observational cohort study in the Netherlands. European Journal of Clinical Pharmacology. 72(5): 615-622.
6. Plat A, Penning-van Beest F, Kessabi S, Groot M, Herings R (2009) Change of initial oral antidiabetic therapy in type 2 diabetic patients. Pharmacy World and Science. 31(6): 622-626.
7. O. Natan, K.P. Bouter (2022) Metformin Intolerance: A Proposal for Defining Using the GSRS. American Journal of Clinical and Experimental Medicine. 10(2): 59-62.
